HKUST researchers Develop an Innovative Microscope Platform to Unveil the Intricacies of Skeletal Muscle Regeneration
Researchers at the Hong Kong University of Science and Technology (HKUST) have created a cutting-edge platform consisting of a dual-laser nonlinear optical microscope to investigate the dynamics of muscle satellite cells (MuSCs) during the process of muscle regeneration. This breakthrough has identified new mechanisms of MuSC behavior in muscle repair, paving the way for the development of targeted therapeutic strategies for muscle-related disorders.
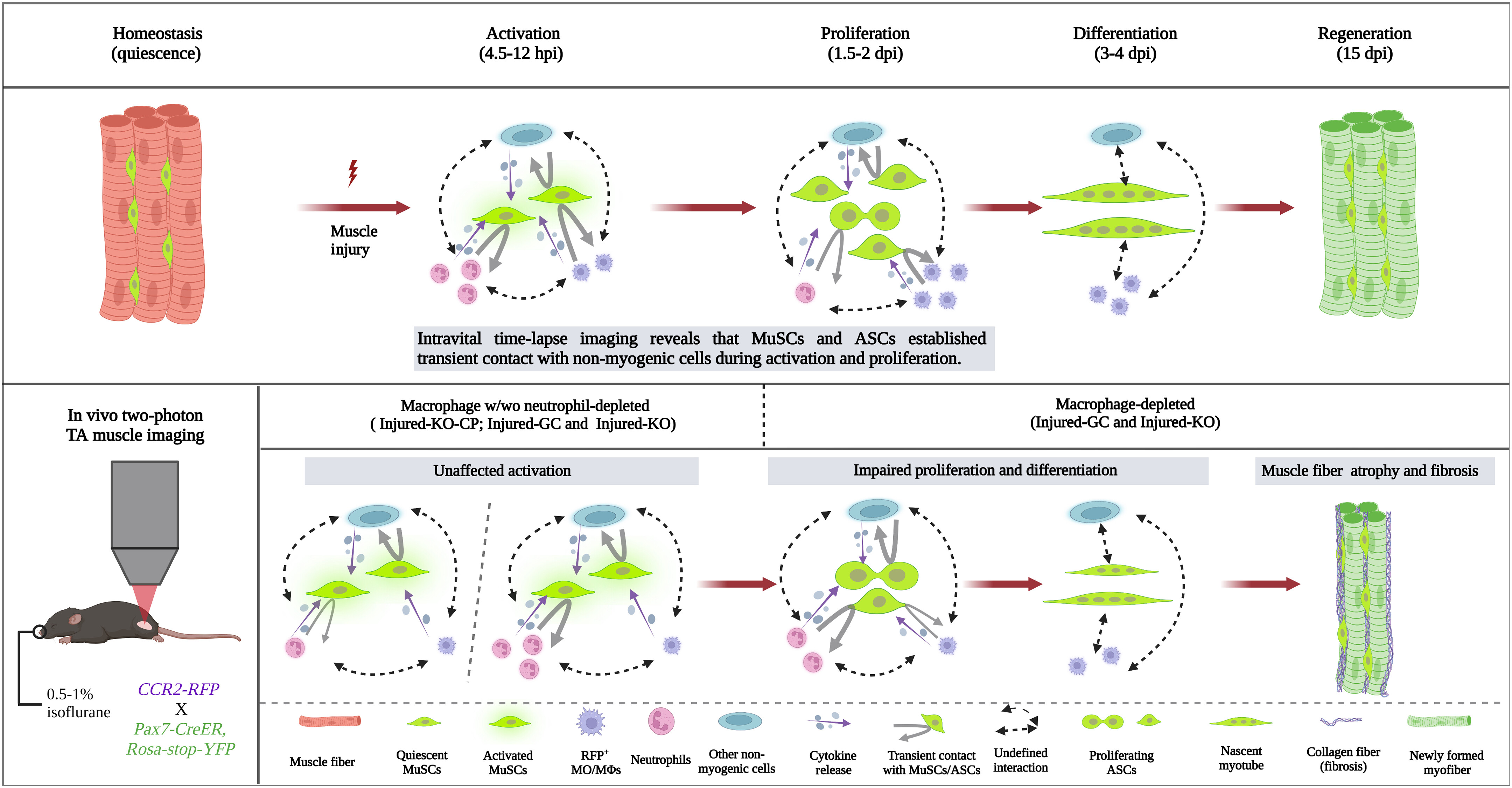
The process of regenerating skeletal muscle relies on the intricate collaboration between MuSCs and various cellular elements. During muscle injury, myeloid cells migrate to the wound alongside the activation of MuSCs. Previous research revealed the morphological heterogeneity of quiescent MuSCs within the muscle microenvironment. These cells establish specialized cellular adhesions and spatial arrangements to maintain their quiescent state. However, the lack of appropriate live animal imaging technology has hindered the analysis of MuSCs' interaction with myeloid cells.
Now, in a collaborative effort, Prof. QU Jianan's team from the Department of Electronic and Computer Engineering at HKUST developed a dual-laser multimodal non-linear optical microscope platform for high-resolution imaging of various cell types and structures within live skeletal muscle, while Prof. WU Zhenguo's team from the Division of Life Science at HKUST contributed their expertise in muscle biology and regenerative processes. Leveraging this innovative imaging technology, their joint research uncovered new insights into the complex processes governing muscle regeneration. Intriguingly, the study challenged the prevailing notion that non-myogenic cells are the primary drivers of MuSC activation. Instead, the team found that MuSCs possess an inherent capacity to sense and respond to regenerative cues independent of external signals from non-myogenic cells.
The study also investigated the role of myeloid cells, particularly macrophages, in regulating MuSC behavior. Macrophages were found to be non-essential for MuSC activation but played a crucial role in their proliferation and differentiation during muscle regeneration. The reduction of macrophages led to impaired cell division and increased fibrosis during the regenerative process, indicating their stage-dependent role in facilitating efficient muscle regeneration.
Additionally, the study explored the real-time interactions between non-myogenic cells and MuSCs. Continuous physical contact between these cell types was not found to be necessary for MuSC activation or cell division. Instead, paracrine signaling from non-myogenic cells appeared to regulate MuSC proliferation, emphasizing the role of secreted factors in coordinating the regenerative processes of MuSCs.
Prof. Qu comments, “Leveraging advanced imaging techniques, this study offers a comprehensive academic exploration of the intricate cellular interactions involved in muscle regeneration, uncovering novel aspects of MuSC behavior. Our findings contribute to the understanding of the complex dynamics underlying muscle regeneration processes and hold significant implications for the development of targeted therapeutic strategies for muscle-related disorders.”
Prof. Qu also highlights the effort of the team led by Prof. WU. Their deep understanding of muscle biology and regenerative processes guided the study's direction and interpretation. They contributed their expertise in designing and conducting experiments on live reporter mice, as well as analyzing the dynamics of MuSCs and their interactions with non-myogenic cells using molecular and cellular biology techniques.
“Prof. Qu’s team contributed their knowledge of optical imaging techniques, laser technology, and computational analysis to create a cutting-edge imaging platform specifically tailored for this study.
The collaboration between the engineering and life science teams in this study represents a multidisciplinary approach to investigating skeletal muscle regeneration. By combining technical skills in advanced imaging technology with biological expertise, we achieved a comprehensive understanding of the intricate cellular interactions involved. This interdisciplinary collaboration fostered creative problem-solving and facilitated the development of novel methodologies and approaches,” said Prof. Wu.
The findings of this collaborative research were recently published in prestigious journal Science Advances.