HKUST Researchers Reveal Microglia’s Crucial Role in Preventing Axonal Degeneration Following Spinal Cord Injury
A collaborative effort between engineers and biologists at the Hong Kong University of Science and Technology (HKUST) has uncovered a neuroprotective mechanism in spinal cord injury (SCI), shedding new light on therapeutic approaches to potentially benefit millions of patients worldwide.
While SCI is a devastating condition with profound, life-altering consequences, unfortunately, a definitive cure remains elusive. For a long time, the study of axon damage and regeneration in SCI has been constrained by the lack of suitable in vivo imaging tools capable of visualizing undisturbed cellular processes within the spinal cord.
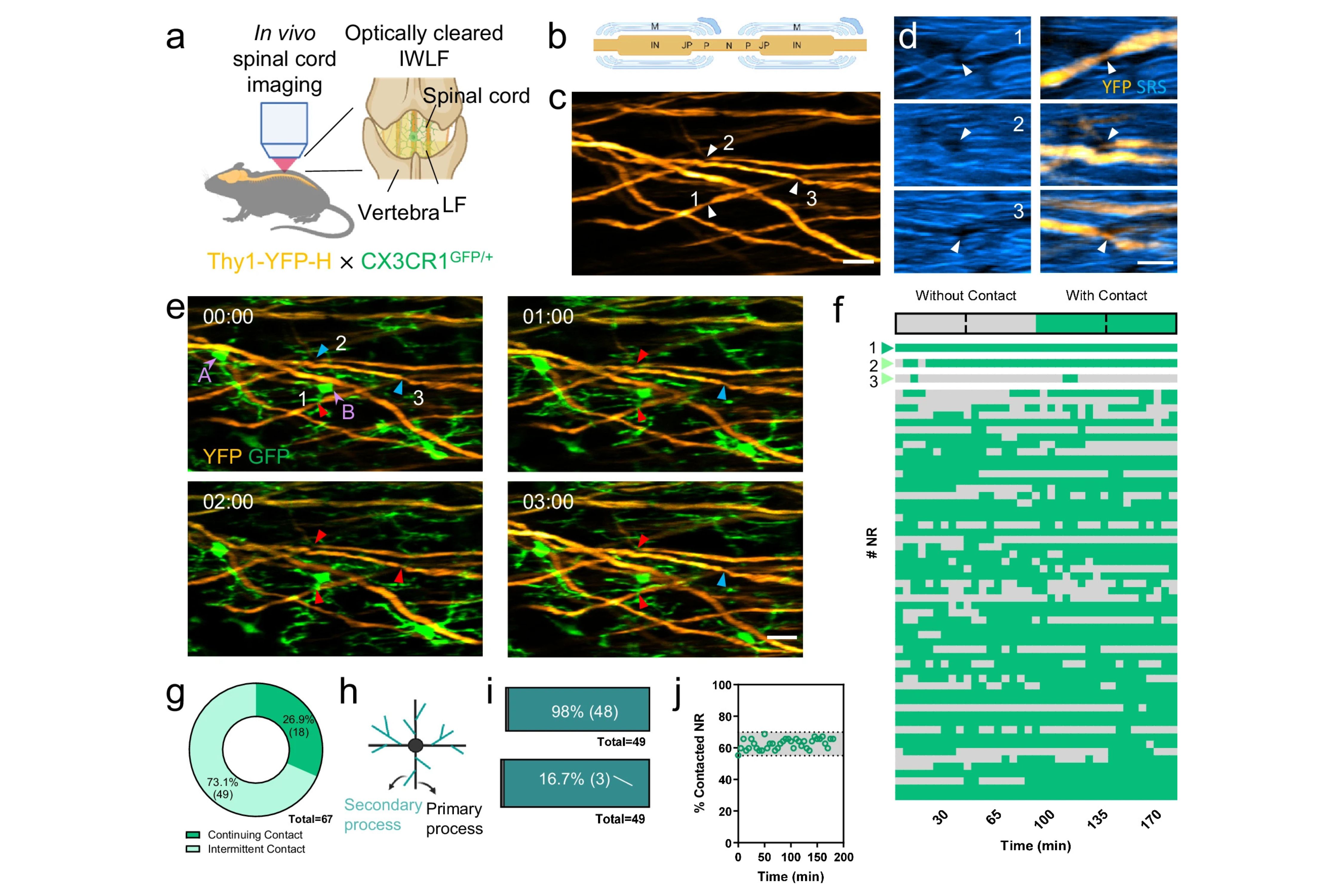
To overcome this hurdle, an HKUST research team co-led by Prof. QU Jianan from the School of Engineering’s Department of Electronic and Computer Engineering (ECE) and Prof. LIU Kai from the School of Science’s Life Science Division, utilized multimodal microscopy and optical clearing technology to enable minimally invasive in vivo imaging studies.
A key to their investigation was to elucidate the critical involvement of microglia in axonal degeneration post-spinal cord axon injury. Microglia are primary immune cells in the central nervous system. They are known to play critical roles in brain development, homeostasis, and neurological disorders. Recent research has underscored the significance of microglial interactions with neurons in processes such as neurogenesis, synaptic plasticity, and neurodegeneration.
Prof. Liu explained, “The present study, facilitated by cutting-edge in vivo multimodal microscopy and optical clearing technology developed in Prof. Qu’s laboratory, showcased the ability to visualize the interaction of glia-nodes in the spinal cord under physiological conditions for the first time.”
“Our findings have unveiled the neuroprotective role of microglia in the acute phase of single spinal cord axon injury. This unique insight into microglia-axon communication offers promising therapeutic targets for effective treatment strategies,” he said.
The team demonstrated that microglia establish direct contact with myelinated axons at nodes of Ranvier in the spinal cord, exhibiting a striking neuroprotective wrapping behavior following axonal injury. This protective mechanism, dependent on the function of P2Y12 receptors, highlights a novel form of neuron-glia interaction that prevents acute axonal degeneration from spreading beyond the nodes.
In addition, they found that voltage-gated sodium channels (NaV) contribute to the interaction between nodes and glial cells following injury, and that the inhibition of NaV can delay axonal degeneration.
Highlighting the significance of this breakthrough, Prof. Qu added, “The collaborative effort between biologists and engineers has led to the discovery of the remarkable functions of microglia during axonal degeneration and regeneration.”
He further pointed out that the study not only illuminates the intricate mechanisms underlying neuronal diseases, but also holds promise for the development of innovative therapeutic approaches in the future.
“The optical imaging platform developed in this cross-disciplinary study will continue to drive our exploration into the mechanisms of various neurological diseases affecting the spinal cord, such as multiple sclerosis, which still remain unclear to date. This will be important for innovating effective treatments that are urgently needed,” he said.
Their findings have recently been published in the prestigious multidisciplinary journal Nature Communications. Dr. WU Wanjie (2023 ECE PhD graduate), HE Yingzhu (ECE PhD student), and CHEN Yujun (Life Science PhD student) are the co-first authors, while Prof. Qu and Prof. Liu are the corresponding authors.