HKUST and PolyU researchers develop an in-vitro vesicle formation assay to reveal mechanistic insights into the secretory pathway
Scientists from The Hong Kong University of Science and Technology (HKUST) and The Hong Kong Polytechnic University (PolyU) have developed an in-vitro vesicle formation assay, shedding light on cargo clients and factors that mediate vesicular trafficking and providing a robust tool to offer novel insights into the secretory pathway.
The secretory pathway is a very important process that takes place in human cells. Many growth factors, hormones and other important factors in the human body are secreted from cells through the secretory pathway to perform their physiological functions. In addition, many newly synthesized proteins must be transported to specific subcellular localization through the secretory pathways to perform their functions. In the secretory transport pathway, transport vesicles function as vehicles to carry cargo molecules. Similar to logistics and delivery services in our daily lives, the transportation of cargo molecules to the correct target sites depends on whether the cargo molecules are accurately sorted into specific transport vesicles. Defects in cargo sorting would induce defects in establishing cell polarity, immunity, as well as other physiological processes.
The key players that mediate protein sorting in the secretory pathway include small GTPases of the Arf family and cargo adaptors. The Arf family GTPases cycle between a GDP-bound inactive state and a GTP-bound active state. Upon GTP binding, Arf proteins mediate membrane recruitment of various cytosolic cargo adaptors. Once recruited onto the membranes, these cargo adaptors recognize sorting motifs on the cargo proteins to package cargo proteins into vesicles.
Although significant progress has been achieved in understanding the general steps of cargo sorting, the spectrum of cargo clients of a specific Arf family member or cargo adaptors remains largely unexplored. Led by Prof. GUO Yusong, Associate Professor of Division of Life Science at HKUST and Prof. YAO Zhongping from PolyU, the research team used an in-vitro assay that reconstitutes packaging of human cargo proteins into vesicles to quantify cargo capture. Quantitative mass spectrometry analyses of the isolated vesicles revealed cytosolic proteins that are associated with vesicle membranes in a GTP-dependent manner. One of them, FAM84B interacts with cargo adaptors and regulates transport of transmembrane cargo proteins. In addition, they uncovered novel cargo proteins that depend on GTP hydrolysis to be captured into vesicles.

Then they utilized this assay and identified cytosolic proteins that depend on a specific Arf family protein, SAR1A, to be recruited to vesicle membranes. One of the cytosolic protein, PRRC1, is recruited to endoplasmic reticulum (ER) exit sites, and interacts with the inner COPII coat. Its absence increases membrane association of COPII and affects ER-to-Golgi trafficking.
Utilizing this assay, they also identified the clients of COPII vesicles. These clients include two cargo receptors, SURF4 and ERGIC53. Through analyzing the protein composition of vesicles isolated from control cells or cells depleted of SURF4 or ERGIC53, they revealed specific clients of each of these two cargo receptors.
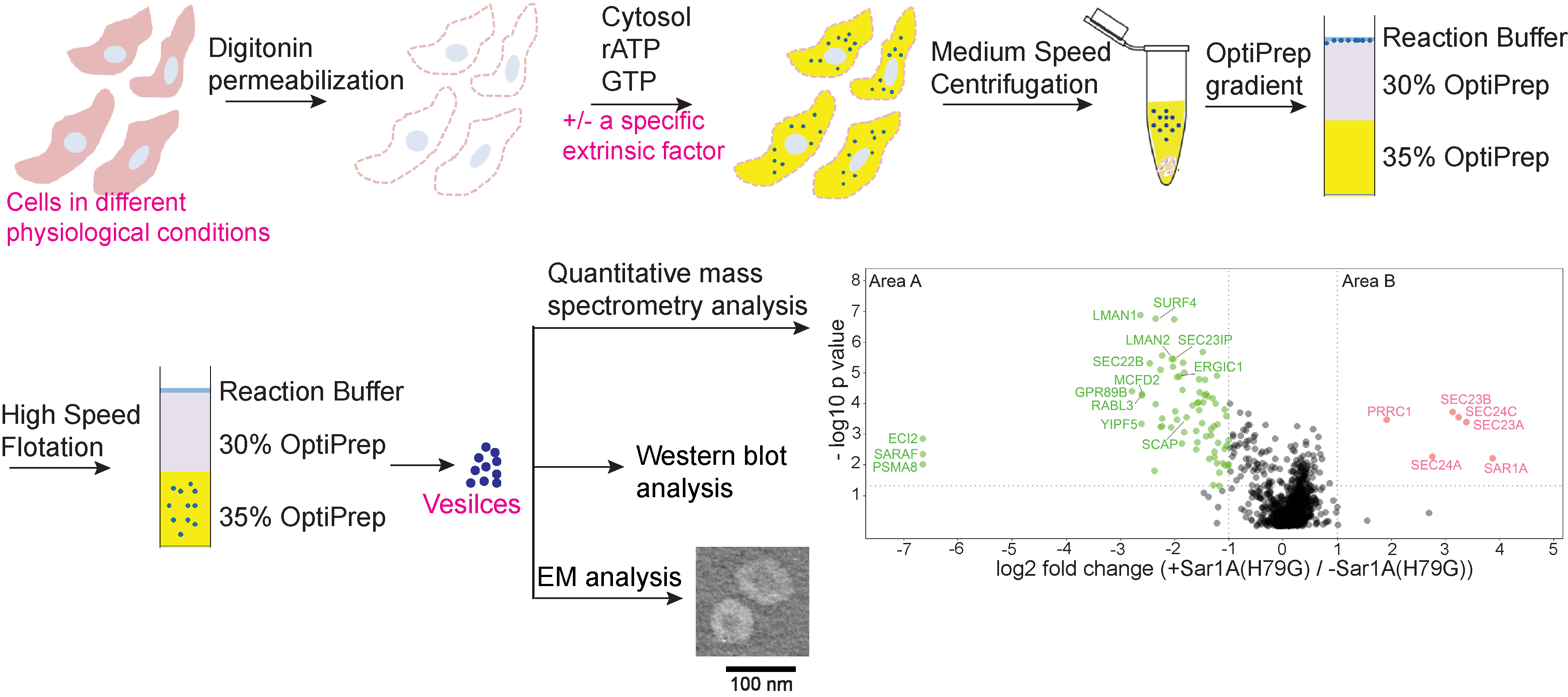
These results indicate that the vesicle formation assay in combination with quantitative mass spectrometry analysis is a robust and powerful tool to systematically reveal cargo proteins that depend on a specific factor to be packaged into vesicles, to analyze protein profiling of transport vesicles under different physiological conditions, and to uncover cytosolic proteins that interact with a specific factor on vesicle membranes.
This study was recently published in Proceedings of the National Academy of Sciences.
Prof. Guo’s research team focuses on investigating molecular mechanisms regulating protein sorting in the secretory pathway. Dr. HUANG Yan (a postdoctoral research associate from Prof. Guo’s lab at HKUST), Dr. YIN Haidi from PolyU and Dr. LI Baiying from CUHK are co-first authors of this study. Mr. LIU Yang, Dr. TANG Xiao, Miss WANG Wo and Miss WU Zhixiao from the Guo lab also participated in this study.










