HKUST Researchers Pioneer Breakthroughs in Lithium-Ion Battery Recycling to Enhance Critical Metal Recovery and Reduce Carbon Emissions
Powering a Circular Battery Economy with Low-Carbon Innovation
Lithium-ion batteries (LIBs) are widely used in consumer electronics, electric vehicles, and renewable energy systems, making efficient recycling crucial for sustainability. A research team led by Prof. Dan TSANG, Professor of Civil and Environmental Engineering at The Hong Kong University of Science and Technology (HKUST), has revealed a previously unrecognized atomic-scale mechanism that obstructs efficient LIB recycling. This breakthrough challenges long-standing assumptions and sets the stage for cleaner, high-yield recovery of critical metals used in LIBs.
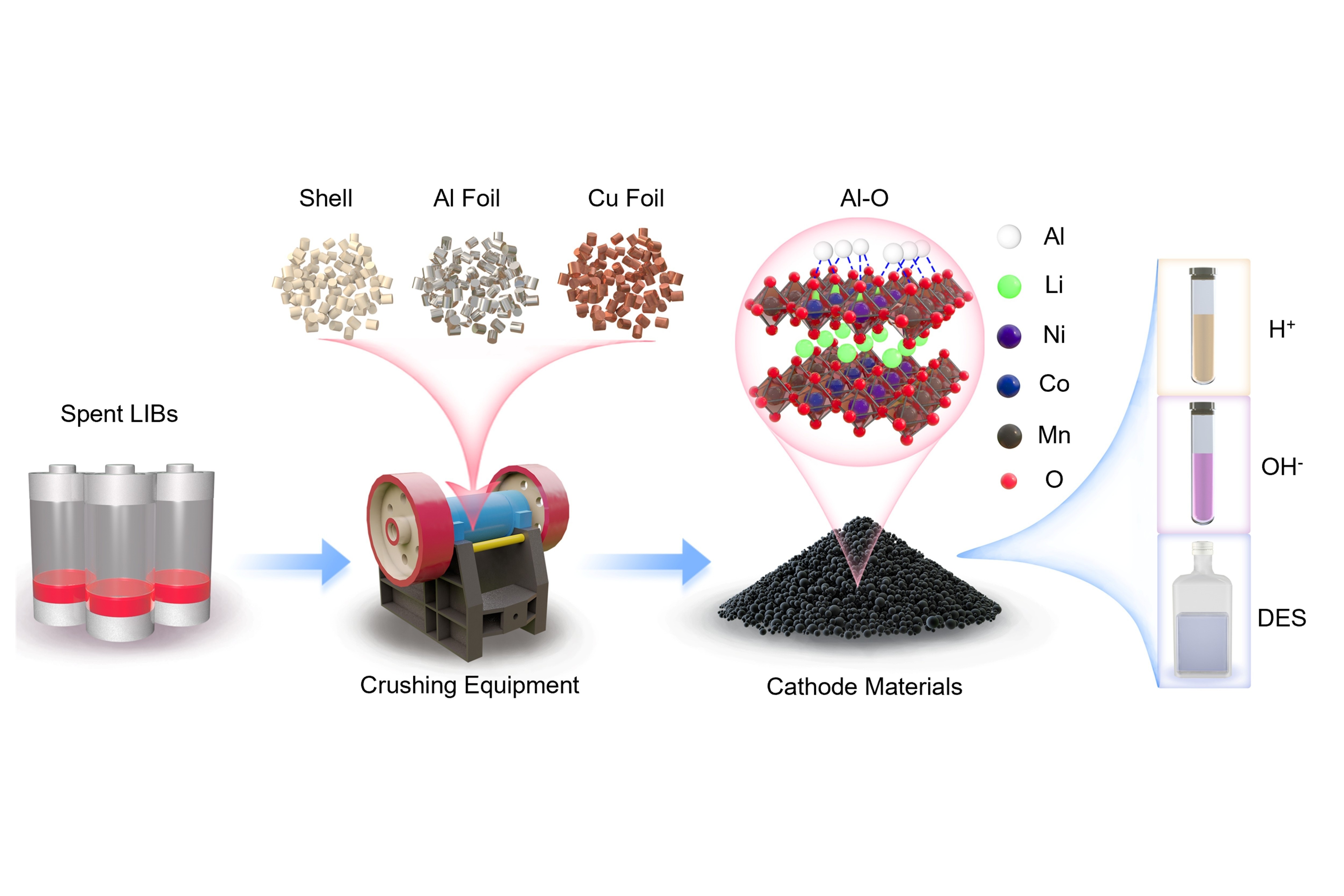
Through advanced characterization and first-principles modeling, the research team found that aluminum (Al) impurities—which come from the mechanical disassembly of LIBs during the recycling process—penetrate NCM (nickel–cobalt–manganese) cathode crystals and restructure the cathodes’ internal chemistry. This triggers the formation of ultra-stable aluminum–oxygen bonds, immobilizing valuable metals and suppressing the metals’ leachability, making extraction more difficult, especially in acidic solvent systems commonly used in hydrometallurgy (the use of water-based solutions to extract metals).
Overlooked Impurities, Underrated Impact: Aluminum as a Hidden Barrier to Recycling
For decades, the presence of aluminum in spent (i.e. used) LIBs has been considered an operational nuisance or a minor issue—now, it has proven to be a mechanistic disruptor that can significantly hinder recycling efforts. The HKUST researchers discovered that during the mechanical disassembly of LIBs, residual aluminum foil can infiltrate NCM (nickel–cobalt–manganese) cathode crystals through frictional contact, subtly but profoundly altering the cathodes’ internal chemistry.
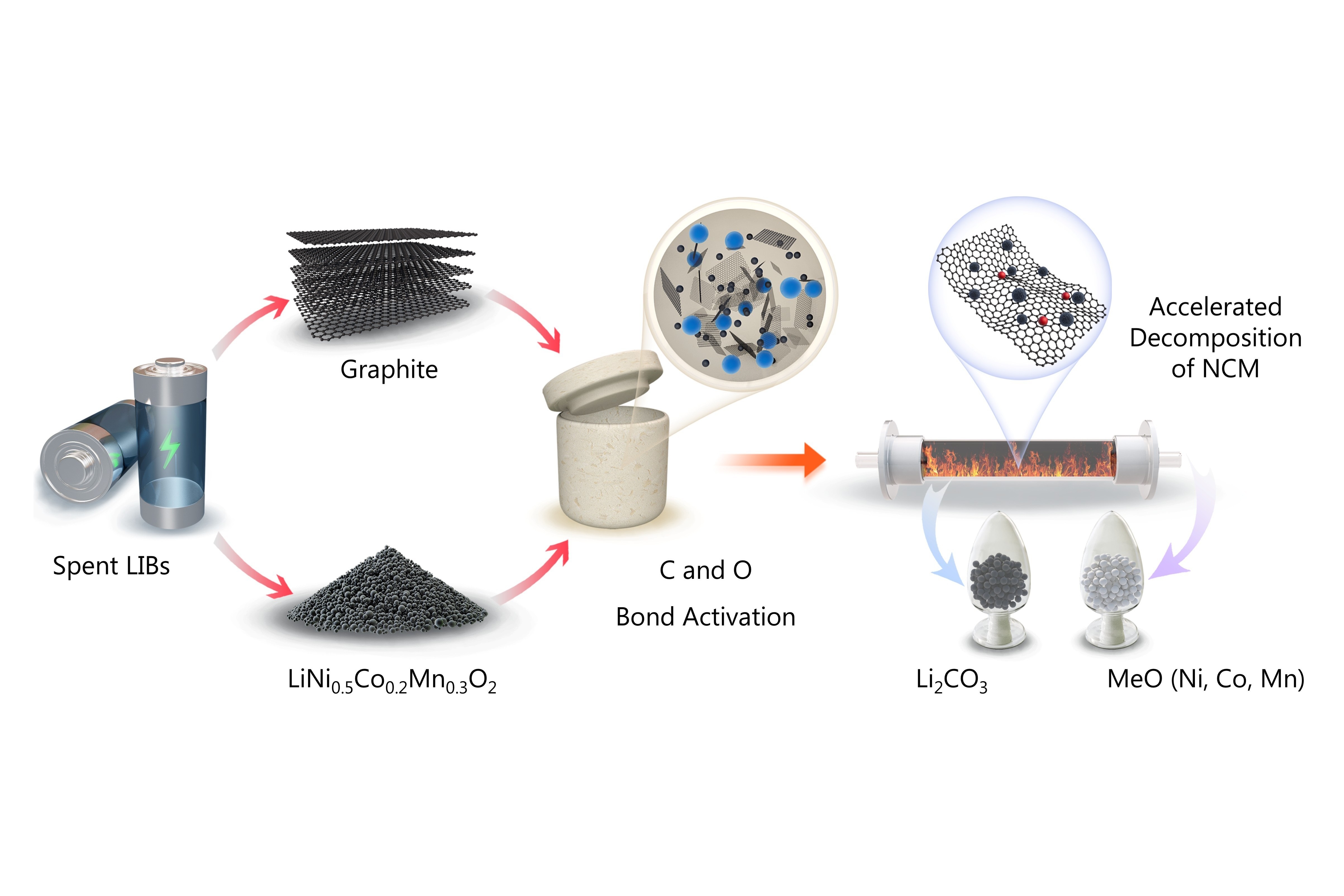
Using advanced microscopy and density functional theory (DFT) modeling, the team found that aluminum atoms selectively replace cobalt, forming highly stable aluminum–oxygen bonds that anchor lattice oxygen and suppress the release of critical metals like nickel (Ni), cobalt (Co), and manganese (Mn) during leaching, making them harder to extract in recycling.
“We’ve shown that even tiny amounts of aluminum contamination can fundamentally shift how NCM materials behave in recycling systems,” said Prof. Tsang. “This demands a paradigm shift in how we manage impurity pathways in battery-to-battery recovery.”
The study further revealed that the types of solvent used in the recycling process affect how aluminum behaves, demonstrating solvent-dependent effects. For example, aluminum slows down metal release in formic acid, enhances it in ammonia, and leads to mixed outcomes in deep eutectic solvents—highlighting the need for precise chemistry-driven process design.
Building the Future of Circular Batteries
Together, these discoveries form a coherent roadmap to overcome two critical bottlenecks in LIB recycling: impurity interference and energy intensity. By combining precision impurity analysis with smart decomposition strategies, the research equips industry and policymakers with the tools needed to scale sustainable battery recovery systems.
“We’re not just solving problems—we’re reframing what efficient, climate-aligned battery recycling looks like,” Prof. Tsang emphasized.
These innovations also align with the United Nations Sustainable Development Goals (SDGs), particularly those focused on responsible consumption and production, affordable and clean energy, and climate action.
Global Collaboration and Scalable Impact
HKUST’s battery recycling research is actively advancing from lab-scale discovery to industrial translation. Led by Prof. Tsang, the team’s findings were recently featured as the back cover of Advanced Science (Volume 12, Issue 21, June 2025), in a paper titled “Dissolution of Spent Lithium-Ion Battery Cathode Materials: Overlooked Significance of Aluminum Impurities”.
Co-authors include Prof. Tsang’s PhD student ZHANG Yuying, former postdoctoral fellows Dr. LIU Kang (now Professor at Qingdao University of Technology) and Dr. WANG Mengmeng (now Hundred Talents Program Research Fellow of the Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences) as well as collaborators at the University of California, Berkeley.
About The Hong Kong University of Science and Technology
The Hong Kong University of Science and Technology (HKUST) (https://hkust.edu.hk/) is a world-class university that excels in driving innovative education, research excellence, and impactful knowledge transfer. With a holistic and interdisciplinary pedagogy approach, HKUST was ranked 3rd in the Times Higher Education’s Young University Rankings 2024, 19th Worldwide and No.1 in Hong Kong in Times Higher Education’s Impact Rankings 2025. Thirteen HKUST subjects were ranked among the world’s top 50 in the QS World University Rankings by Subject 2025, with “Data Science and Artificial Intelligence” holding the 17th place, maintaining its position as first in Hong Kong. Our graduates are highly competitive, consistently ranking among the world’s top 30 most sought-after employees. In terms of research and entrepreneurship, over 80% of our work was rated “Internationally excellent” or “world leading” in the latest Research Assessment Exercise 2020 of Hong Kong’s University Grants Committee. As of May 2025, HKUST members have founded over 1,800 active start-ups, including 10 Unicorns and 17 exits (IPO or M&A).